CONSULTING
Lunch with Abbott SJM Team
SIMPLIFYING COMPLIANCE
We offer distinctive competencies leading technical teams through Design Control, and Product Realization to facilitate compliance and minimize development time. We provide subject matter expertise and guidance to the following processes:
- Guide R/D team through a simplified, gated, FDA/ISO/MDR compliant and performing process
- Speed up Design and Process FMECA
- Validate design significant parameters to their appropriate sigma levels
- Validate Process capability to designed sigma levels
- Validate existing test methods, and measurements processes
- Review and simplify quality procedures
- Qualify suppliers to meet the required capability, and establish vendor surveillance to ensure sustained capability
- Audit DHF, 510k/PMA/Technical File, Risk Management, and associated deliverables for communicability and compliance
- Coach management on how reducing variations reduces cost.
GUIDING FDA/ISO/EU MDR 2017
With over 20 years of experience implementing a closed loop feedback system for product lifecycle management, we have gained a profound knowledge of the regulatory trends, and will put your team ahead of the FDA/ISO/MDR compliance curve.
Lunch with Edwards'Team
Partial list of customers and projects we have supported
Slide title
FMS InfuLife™ Infusion Pump
Button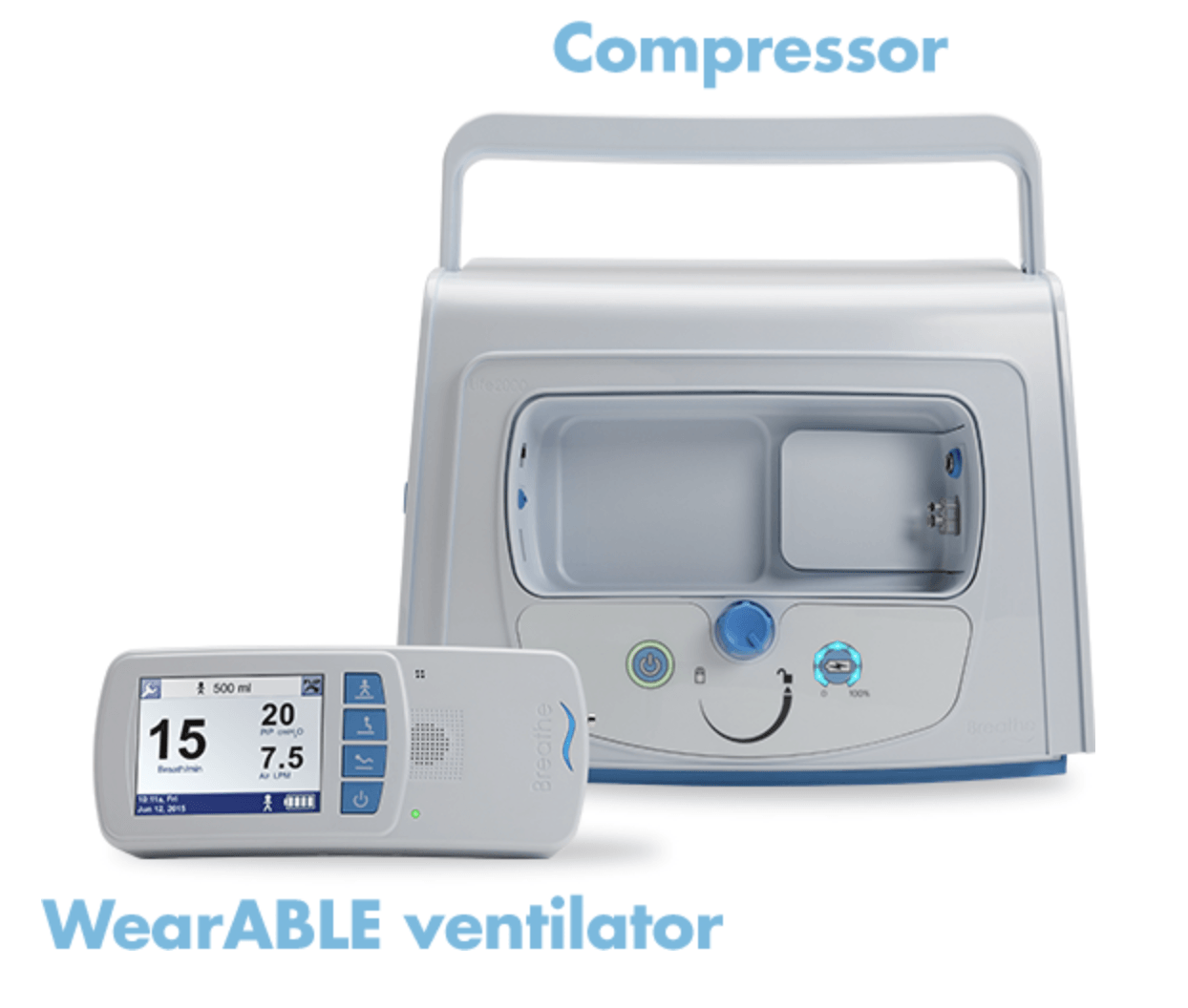
Breathe Technologies Ventillator
Button
Abbott LVAD
Button
Optiscan Bedside Glucose Monitor
Button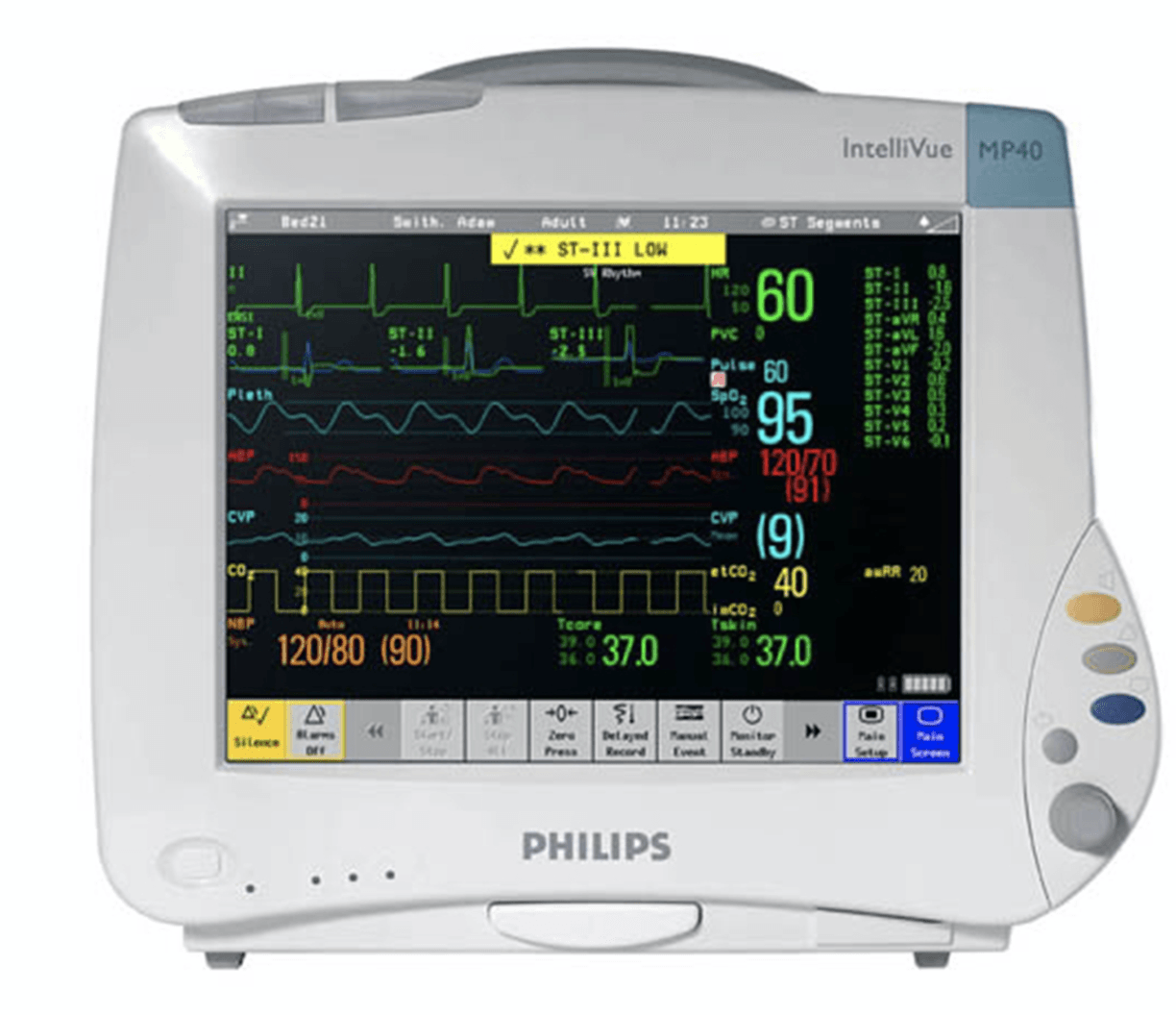
Philips Patient Monitor
Button
Philips Defibrillator
Button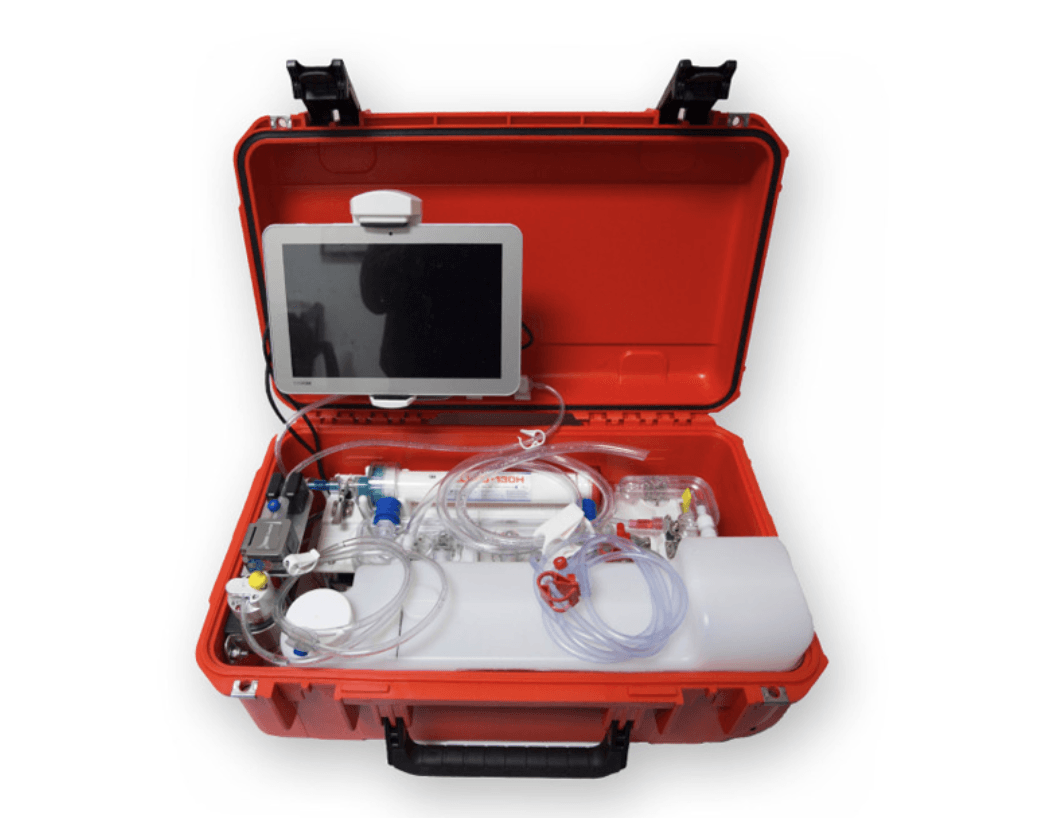
Easy Dial Portable Hemodialysis System
Button
Edwards S3 Heart Valve
Button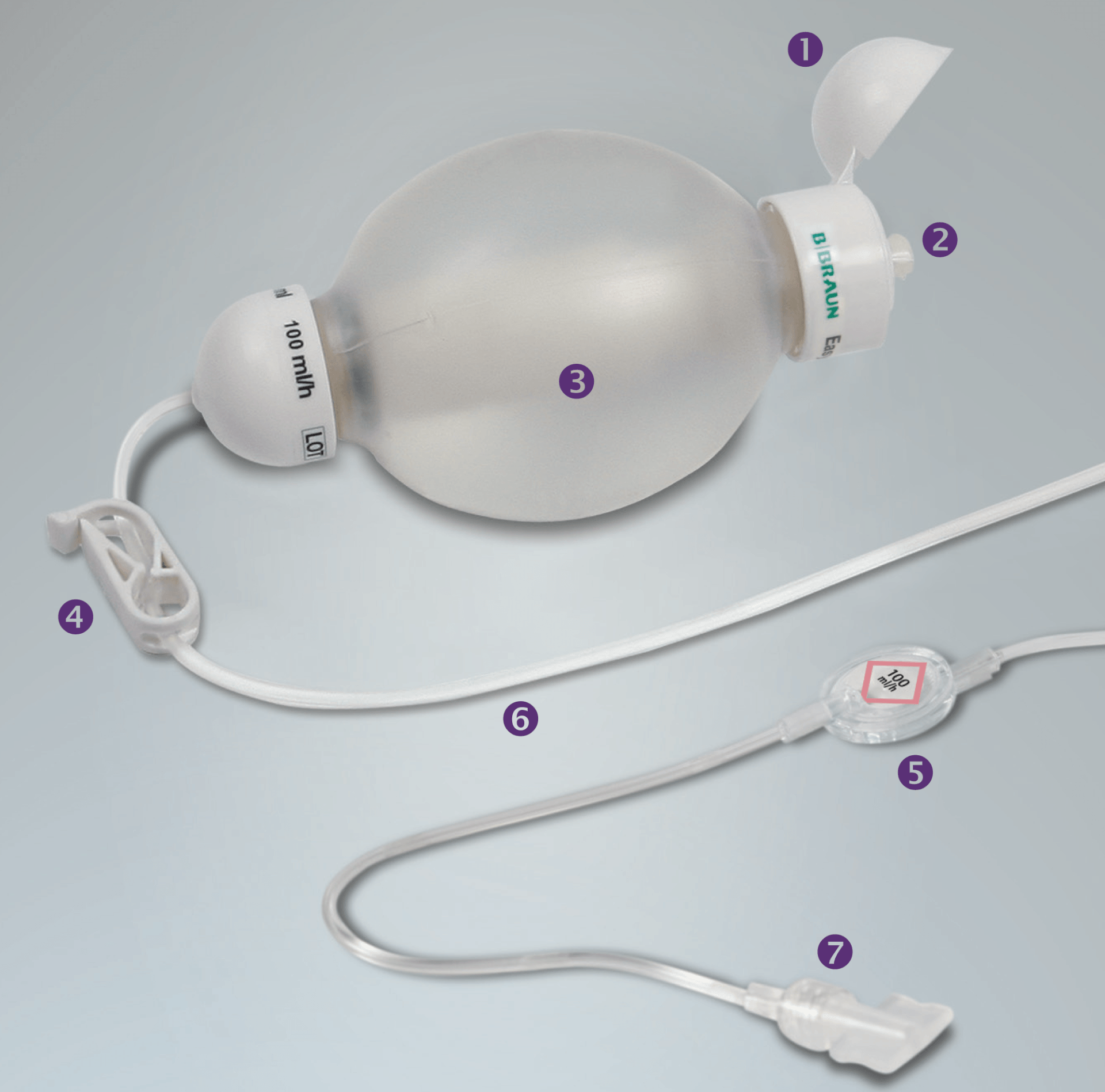
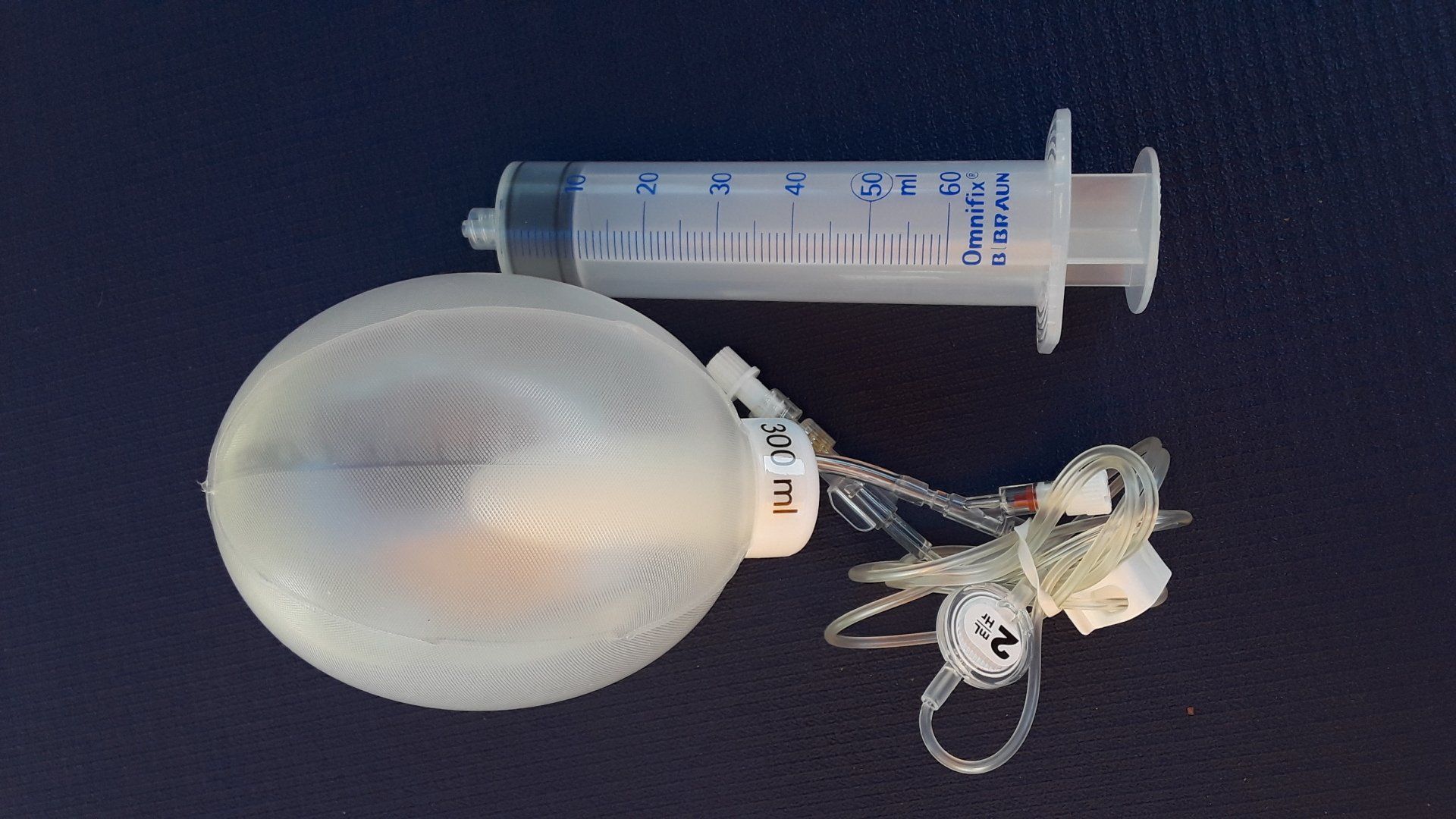
B Braun Easypump
Button
Slide title
McKinley Beeline Infusion Pump
Button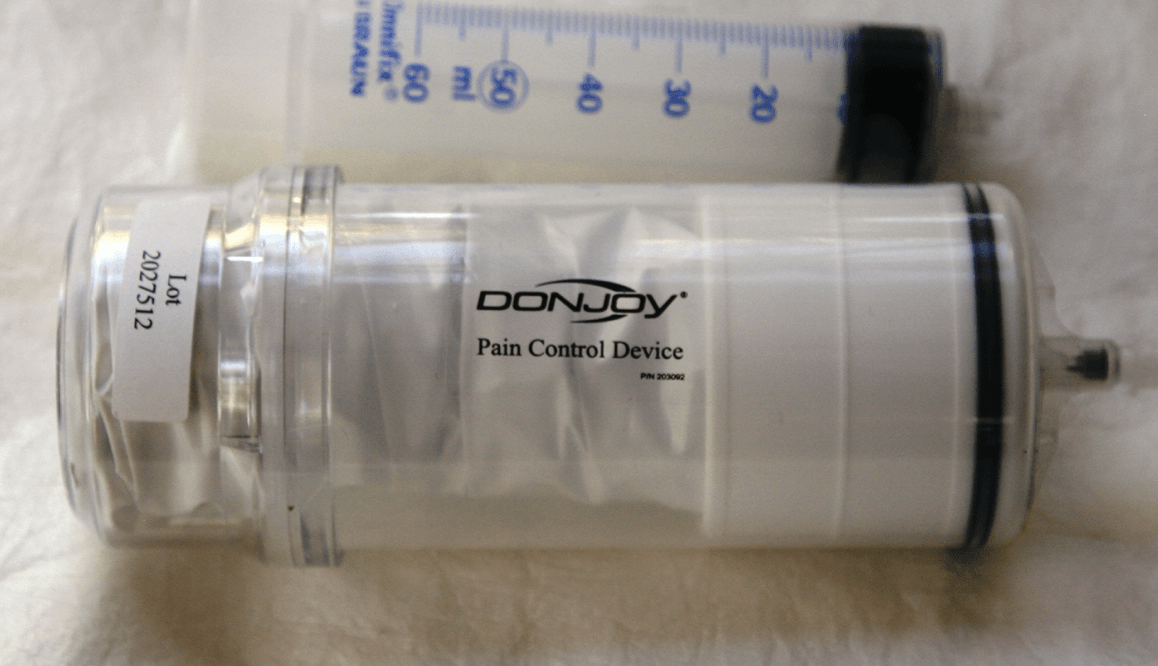
Slide title
DonJoy Pain Management Kit
ButtonCurlin Infusion Pump
Button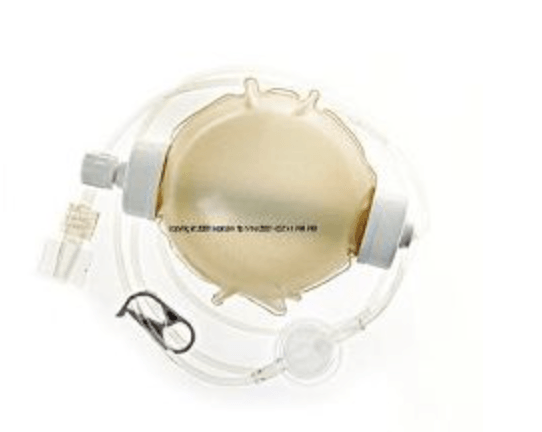
Iflow Eclipse Pump
Button
FIRST MEDICAL SOURCE

