Revolutionizing Infusion Therapy
Introducing InfuLife®: The Next Generation of Elastomeric Pumps

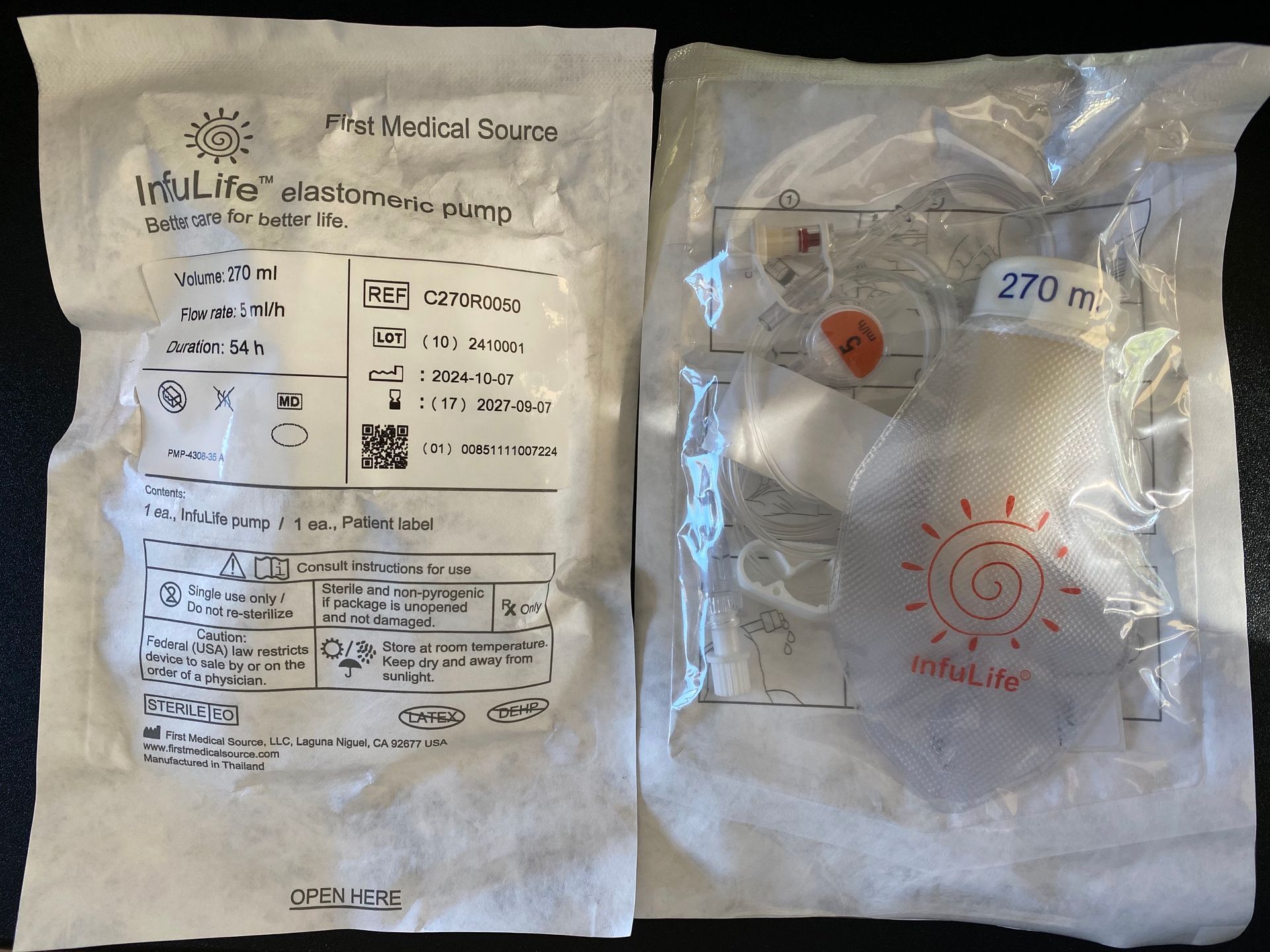


InfuLife® pumps
FMS is proud to offer InfuLife® the next generation of elastomeric infusion pump.
Specially designed to eliminate known problems of earlier generations, InfuLife® is 40% more accurate, requires half filling effort, and is 50% less likely to leak than the previous generations of elastomeric pumps.
These characteristics, preventing spillage, over/under infusions, waste of time, complaints and returns make InfuLife® ideal of chemotherapy and pain management.
Filling InfuLife
Priming InfuLife
InfuLife® is
- indicated for infusion of antibiotics, chemotherapy and pain management
- Not made with natural rubber, Latex or DEHP
- Easy to fill
- Fill-Volumes up to 400 mL
- Infusion rates 0.5 to 200 ml/hr.
- Accuracy:
- +/- 12% for chemo and pain pumps (flow rates .5 to 10 mL/h)
- +/- 9% for antibiotic pumps (flow rates 50 to 200 mL/h).
FMS ANESTHESIA KITS
Double Site Anesthesia Kit, FMS Conduction Catheter 5" REF YC50
Each Catheter Pack contains:
- 2 ea 5" Fenestrated FMS Conduction Catheter™
- 2 ea 18G x 4.5" Tear Away Introducer Needle
- 2 ea Securement Dressing
- 2 ea Adhesive Strip Cards
- 2 ea Priming/Flush Syringe
- 1 ea Filling Syringe
- 2 ea Non-Vented Cap
- 1 ea Bifurcated Set
- 1 ea Instructions for Use
CAUTION: FMS pumps are contraindicated for intra-articular use. There is an inherent risk in using any medical device. Please refer to the product labeling for Indications, Cautions, Warnings and Contraindications. Failure to follow the product labeling may directly impact patient safety. Physicians are responsible for prescribing and administering medications per instructions provided by the drug manufacturers.
InfuLife® is cleared for distribution in the USA under the 510k K240624.
Federal law restricts these devices to sale by or on the order of a licensed healthcare practitioner.

